- IPCR team (location: Calais)
Leica STELLARIS 5 confocal microscope
 The principle of confocal laser scanning microscopy is to produce optical sections for three-dimensional imaging of biological tissues. The Stellaris 5 allows the spectral emission band to be adapted to all types of fluorescent probe. It offers spectral scanning to characterise a fluorophore or to separate two probes with overlapping spectra. It is equipped with four lasers to cover all visible and near-IR fluorophores. Its three detectors enable simultaneous acquisition of three fluorescence channels, while the fourth detector is dedicated to bright field imaging (DIC).
The principle of confocal laser scanning microscopy is to produce optical sections for three-dimensional imaging of biological tissues. The Stellaris 5 allows the spectral emission band to be adapted to all types of fluorescent probe. It offers spectral scanning to characterise a fluorophore or to separate two probes with overlapping spectra. It is equipped with four lasers to cover all visible and near-IR fluorophores. Its three detectors enable simultaneous acquisition of three fluorescence channels, while the fourth detector is dedicated to bright field imaging (DIC).
Common applications:
- 3D imaging
- Colocation
- Signal quantification
- Microscope also designed for F-techniques (FRAP, FRET)
Acquisition modes :
- Multi-channel confocal microscopy
- 3D reconstruction, XZ scan
- Spectral imaging, spectral separation
- Timelaps (integrated autofocus management)
- Multiposition, mosaics
- Bright field imaging DIC (differential interference contrast)
- Fluorescence filter cubes: DAPI, GFP and Rhodamine
Microscope :
Leica DMi8 inverted stand
Motorised XY stage Super Z galvanometric stage for samples fixed between slide and coverslip or live samples on slide, Petri dish or multi-well plate.
Lenses:
- 4x HC PL FLUOTAR 4x/0.13
- 10x HC PL APO 10x/0.40 CS2 (working distance 2.56mm)
- 20x HC PL APO 20x/0.75 CS2 (working distance 0.62mm)
- 16x HC FLUOTAR L 16x/0.6 IMM (working distance 2.5mm)
- 63x HC PLA APO 63x/1.40 Oil CS2 (working distance 0.14mm)
Lasers :
- 405nm laser (50mW)Laser 488nm (20mW)
- Laser 561nm (20mW)
- Laser 638nm (30mW)
Detectors :
- 3 spectral HyD S detectors
- 1 Detector for transmitted light
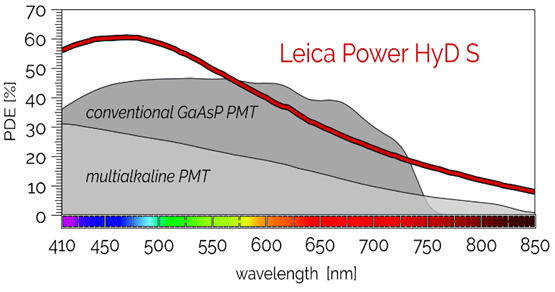
The design of the HyD detectors provides increased sensitivity and signal-to-noise ratio compared with a conventional PMT and low-noise photon-counting imaging.
Scanners :
Conventional scanner (10im/s) and Resonant scanner (28im/s)
Software :
Control software including an adaptive deconvolution module (LIGHTNING technology) and a mosaic acquisition module.
Applications:
Thanks to the development of a wide variety of proteins and fluorescent probes, it is now possible to mark and visualise virtually any molecule or structure in animal or plant tissues or cells. This makes it possible to monitor complex biological processes such as mitosis, changes in the cytoskeleton, the accumulation of molecules at cellular level, etc., and also to answer questions about molecular dynamics and interactions on living biological material.
This equipment is combined with a semi-automatic rotating microtome (LEICA HistoCore MULTICUT R) for producing reproducible, uniform paraffin sections and a vibrating blade microtome (Leica VT1200) designed for sections of fixed or unfixed samples.
Illustrations :
THREE TRINOCULAR OPTICAL MICROSCOPES
 OLYMPUS BX40 (10X 20X 40X 100X) direct
OLYMPUS BX40 (10X 20X 40X 100X) direct- OLYMPUS CKX 41 (10X-40X) inverted
- NIKON Eclipse E600 with fluorescence (FITC / DAPI) and phase contrast (10X 20X 40X 50X 100X)
HIGH-RESOLUTION COLOUR IMAGE & VIDEO ACQUISITION SYSTEM ADAPTED TO FLUORESCENCE
- Olympus DP74 camera
- Low noise fluorescence CMOS colour sensor
- Max resolution: 5760 x 3600 pixels
- 60 fps video
- Olympus CellSens software
WinRHIZOTM
Image analysis system (scanner & software) in its “Regular” version for studying the morphology of plant root systems.
Features (“REG” version), automated morphological measurements:
Global measurements (on image)
- Number
- Total length
- Average diameter
- Total area
- Number of main and secondary roots
Refined measurements
- Determination of the proportions (%) of thick vs fine roots (according to diameter)
- For each class defined :
- Number
- Length
- Surface
- Volume
Possibility of manual ETC nodule counting.
Location: UCEIV Calais
Portable system for measuring photosynthetic activity, transpiration, stomatal conductance, internal CO2 and vapour pressure differential on a leaf.
Features :
- High-precision infrared CO2 and H2O gas analyser
- 1 Chlorophyll Fluorescence and LED light module for simultaneousmeasurements of Photosynthesis and Chlorophyll Fluorescence
- 1 PAR (Photosynthetically Active Radiation) sensor
- 1 infrared temperature sensor in the cuvette under the leaf for non-contact measurement of leaf temperature
- 1 temperature sensor in the cuvette to measure and regulate temperature
- 1 pressure sensor to measure atmospheric pressure
- 1 Universal clamp with 3 interchangeable windows (18mm diameter/2.5cm2) adapted to different leaf sizes
- 1 Chamber for measuring soil respiration
Applications :
Assessment of the state of health of a plant subjected to biotic or abiotic stress by measuring leaf gas exchange and photosynthetic activity. An optional module can also measure soil respiration.
Location: UCEIV Calais
1 Growing room
Cultivation in semi-controlled conditions
- Evenly regulated temperature
- Fixed illuminance, alternating day and night
12 MLR-352-H climate chambers
Cultivation under PiD microprocessor-controlled conditions
- Programmable temperatures from 0°C to 50°C over 12 stages
- Programmable illuminance from 0 to 20,000 lux
- Relative humidity adjustable from 55% to 90%.
Location: UCEIV Calais
- Real-Time PCR Detection Systems : BIO-RAD
 Features :
Features :
Gene expression analysis software: CFX ManagerTM for rapid analysis of the relative expression of differentially expressed genes. Protocol creation wizard and assistance in choosing household genes suited to the matrices being worked on. The device contains a block of 96 wells.
- Thermocyleur : SureCycler 8800 Agilent®
 Features :
Features :
The device contains a 96-well block, with reaction volumes ranging from 10-100µL. 10,000 protocol memory capacity, temperature rise of 6°C/sec. Intra-block gradient range from 30 to 99°C, enabling optimisation of primer pairs designed in the laboratory.
Applications :
All these devices can be used to monitor the expression of genes of interest linked to the state of health of a plant subjected to biotic or abiotic stress under controlled conditions (temperature, light, humidity).
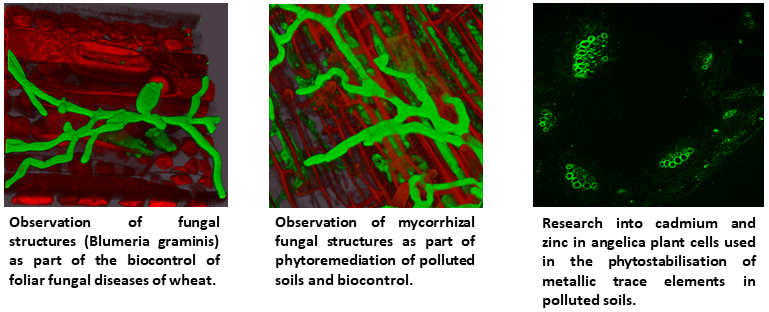
Our team has developed expertise in the responses of common wheat (Triticum aestivum L.) to :
– Inoculation with pathogenic fungi (Zymoseptoria tritici or Blumeria graminis f.sp. tritici),
– Inoculation with beneficial organisms (mycorrhizal fungi)
– Spraying with elicitors from various sources (algae, bacteria, fungi, higher plant extracts, organic acids, etc.).
Location: UCEIV Calais
Anton Paar Multiwave 3000 modular microwave system
Microwave-assisted extraction offers a number of advantages over conventional techniques, such as shorter extraction times, higher extraction yields and reduced use of solvents.
The system allows precise wireless measurement and control of the internal temperatures and pressures of the reactors.
Thanks to its 16 independent reactors, it limits contamination and ensures high process reproducibility.
Some examples of applications for microwave-assisted sample preparation:
-Acid digestion for extracting trace elements from plant or soil matrices
-Solid/liquid (solvent) extraction of organic molecules such as pollutants (dioxins/furans, alkanes and PAHs) from plant matrices or soils
-Accelerated evaporation / concentration (acid / aqueous) of samples
Location: UCEIV Calais
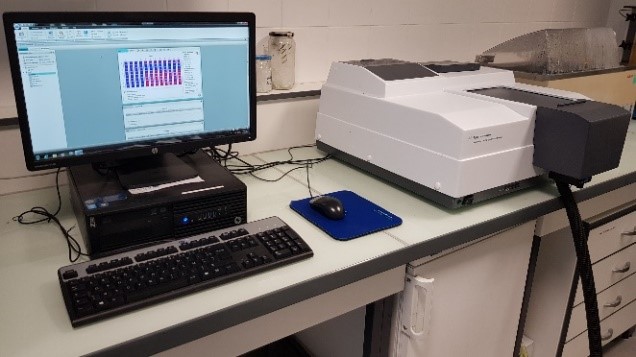
- Spectrophotometer AGILENT CARY 100
 Specifications :
Specifications :
- Dual-beam UV – Visible (Tungsten – Halogen) spectrophotometer with a working range above 4.0 Abs.
- Records absorption and/or transmission spectra over the 190 nm – 900 nm spectral range (bandwidth 0.20-4.00 nm).
- Multicell 6×6 Peltier cells support unit and temperature controller (-10°C – 100 °C)
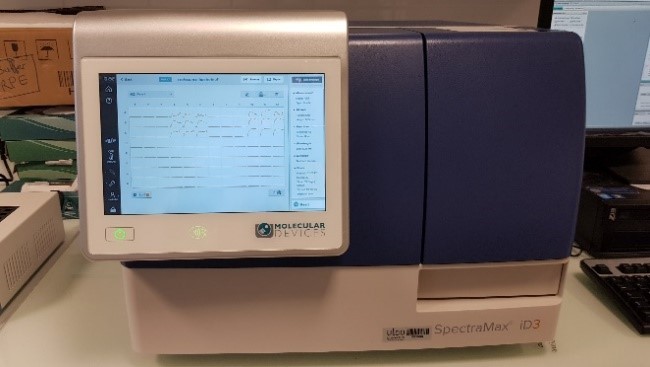
- SpectraMax® iD3 multimode microplate reader
 Specifications :
Specifications :
- Measures Absorbance, FLuorescence and Luminescence
6 to 384 wells – double orbital linear shaking - Temperature control: +5°C to +66°C
- Abs: 230 to 1000 nm – FL excitation: 250 to 830 nm – FL emission: 270 to 850 nm – Lumi: 300 to 850 nm
Some examples of applications:
- Measurement of bacterial and fungal growth
- Measurement of enzymatic activities: peroxidase, catalase, Glutathione S-transferase, etc.
- Measurement of the anti-inflammatory and antioxidant activities of essential oils
- Quantification of DNA extracted from soils or plant or fungal material
Detection and quantification of proteins - Quantification of compounds extracted from plants: total phenolic compounds, total chlorophylls and carotenes, etc.
Location: UCEIV Calais

